Bioassay : Its importance and methology
Bioassay is defined as the estimation of the potency of an active principle in a unit quantity of preparation or detection and measurement of the concentration of the substance in a preparation using biological methods (i.e. observation of pharmacological effects on living tissues, microorganisms or immune cells or animal). Hence micro bioassay, radioimmunoassay are also regarded as `bioassay'. Recently `biotechnology' has also been considered for bioassay. Bioassay of the products like erythropoietin, hepatitis- B vaccine etc. is being done through biotechnology.
Importance of Bioassay
Bioassays, as compared to other methods of assays (e.g. chemical or physical assay) are less accurate, less elaborate, more laborious, more troublesome and more expensive. However, bioassay is the only method of assay if
(1) Active principle of drug is unknown or cannot be isolated, e.g. insulin, posterior pituitary extract etc.
(2) Chemical method is either not available or if available, it is too complex and insensitive or requires higher dose e.g. insulin, acetylcholine.
(3) Chemical composition is not known, e.g. long acting thyroid stimulants.
(4) Chemical composition of drug differs but have the same pharmacological action and vice-versa, e.g. cardiac glycosides, catecholamines etc.
Moreover, even if chemical methods are available and the results of bioassay conflict with those of the chemical assay, the bioassay is relied upon and not the chemical assay, since it is the assessment on living organism.
The purpose of bioassay is to ascertain the potency of a drug and hence it serves as the quantitative part of any screening procedure (Research). Other purpose of bioassay is to standardize the preparation so that each contains the uniform specified pharmacological activity. In this way, it serves as a pointer in the Commercial Production of drugs when chemical assays are not available or do not suffice. From the clinical point of view, bioassay may help in the diagnosis of various conditions, e.g. gonadotrophins for pregnancy.
Principle of Bioassay
The basic principle of bioassay is to compare the test substance with the International Standard preparation of the same and to find out how much test substance is required to produce the same biological effect, as produced by the standard. The standards are internationally accepted samples of drugs maintained and recommended by the Expert Committee of the Biological Standardization of W.H.O. They represent the fixed units of activity (definite weight of preparation) for drugs. In India, standard drugs are maintained in Government institutions like Central Drug Research Institute, Lucknow, Central Drug Laboratory, Calcutta, etc.
The problem of biological variation must be minimized as far as possible. For that one should keep uniform experimental conditions and assure the reproducibility of the responses.
Methods of Bioassay for Agonists
An agonist may produce graded response or quantal response. Graded response means that the response is proportional to the dose and response may lie between no response and the maximum response. By quantal, it is meant that the response is in the form of "all or none", i.e. either no response or maximum response. The drugs producing quantal effect can be bioassayed by end point method. The drugs producing graded responses can be bioassayed by (1) Matching or bracketing method or
(2) Graphical method.
1. End Point Method: Here the threshold dose producing a positive effect is measured on each animal and the comparison between the average results of two groups of animals (one receiving standard and other the test) is done. e.g. bioassay of digitalis in cats. Here the cat is anaesthetized with chloralose and its blood pressure is recorded. The drug is slowly infused into the animal and the moment the heart stops beating and blood pressure falls to zero, the volume of fluid infused is noted down. Two series of such experiments-one using standard digitalis and the other using test preparation of digitalis is done.
In case, if it is not possible to measure individual effective dose or if animals are not available, fixed doses are injected into groups of animals and the percentage of mortality at each dose level is determined. The percentage of mortality is taken as the response and then the comparison is done in the same way as done for graded response.
2. Matching Method: In this method a constant dose of the test is bracketed by varying doses of standard till the exact match is obtained between test dose and the standard dose.
Initially, two responses of the standard are taken. The doses are adjusted such that one is giving response of approximately 20% and other 70% of the maximum. The response of unknown which lies between two responses of standard dose is taken. The panel is repeated by increasing or decreasing the dose s of standard till all three equal responses are obtained. The dose of test sample is kept constant. At the end, a response of the double dose of the standard and test which match each other are taken. These should give equal responses.
This method has following limitations:
1. It occupies a larger area of the drum as far as tracings are concerned.
2. The match is purely subjective, so chances of error are there and one cannot determine them.
3. It does not give any idea of dose-response relationship.
However, this method is particularly useful if the sensitivity of the preparation is not stable. Bioassay of histamine, on guinea pig ileum is preferably carried out by this method.
3. Graphical method: This method is based on the assumption of the dose-response relationship. Log-dose-response curve is plotted and the dose of standard producing the same response as produced by the test sample is directly read from the graph. In simpler design, 5-6 responses of the graded doses of the standard are taken and then two equiactive responses of the test sample are taken. The height of contraction is measured and plotted against the log-dose. The dose of standard producing the same response as produced by the test is read directly from the graph and the concentration of test sample is determined by the same formula as mentioned before.
The characteristic of log-dose response curve is that it is linear in the middle (20-80%). Thus, the comparison should be done within this range only. In other words, the response of test sample must lie within this range.
Advantage of this method is that, it is a simple method and chances of errors are less if the sensitivity of the preparation is not changed. Other methods which are based on the dose-response relationship include 3 point, 4 point, 5 point and 6 point methods. In these methods, the responses are repeated several times and the mean of each is taken. Thus, chances of error are minimized in these methods. In 3 point assay method 2 doses of the standard and one dose of the test are used. In 4 point method 2 doses of standard and 2 doses of the test are used. In 6 point method 3 doses of standard and 3 doses of the test are used. Similarly one can design 8 point method also. The sequence of responses is followed as per the Latin square method of randomization in order to avoid any bias.
The mean responses are calculated and plotted against log-dose and amount of standard producing the same response as produced by the test is determined graphically as well as mathematically.
Bioassay of Antagonists:
Commonly used method for the bioassay of antagonist is simple graphical method. The responses are determined in the form of the percentage inhibition of the fixed dose of agonist. These are then plotted against the log dose of the antagonist and the concentration of unknown is determined by finding out the amount of standard producing the same effect as produced by the test.
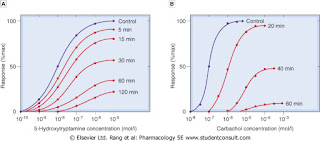
In this method, two responses of the same dose of agonist (sub maximal giving approximately 80% of the maximum response) are taken. The minimum dose of standard antagonist is added in the bath and then the response of the same dose of agonist is taken in presence of antagonist. The responses of agonist are repeated every ten min till recovery is obtained. The higher dose of standard antagonist is added and responses are taken as before. Three to four doses of the standard antagonist are used and then one to two doses of test sample of the antagonist is used similarly. The percentage inhibition is calculated, plotted against log dose of antagonist and the concentration of unknown is determined as usual.
Importance of Bioassay
Bioassays, as compared to other methods of assays (e.g. chemical or physical assay) are less accurate, less elaborate, more laborious, more troublesome and more expensive. However, bioassay is the only method of assay if
(1) Active principle of drug is unknown or cannot be isolated, e.g. insulin, posterior pituitary extract etc.
(2) Chemical method is either not available or if available, it is too complex and insensitive or requires higher dose e.g. insulin, acetylcholine.
(3) Chemical composition is not known, e.g. long acting thyroid stimulants.
(4) Chemical composition of drug differs but have the same pharmacological action and vice-versa, e.g. cardiac glycosides, catecholamines etc.
Moreover, even if chemical methods are available and the results of bioassay conflict with those of the chemical assay, the bioassay is relied upon and not the chemical assay, since it is the assessment on living organism.
The purpose of bioassay is to ascertain the potency of a drug and hence it serves as the quantitative part of any screening procedure (Research). Other purpose of bioassay is to standardize the preparation so that each contains the uniform specified pharmacological activity. In this way, it serves as a pointer in the Commercial Production of drugs when chemical assays are not available or do not suffice. From the clinical point of view, bioassay may help in the diagnosis of various conditions, e.g. gonadotrophins for pregnancy.
Principle of Bioassay
The basic principle of bioassay is to compare the test substance with the International Standard preparation of the same and to find out how much test substance is required to produce the same biological effect, as produced by the standard. The standards are internationally accepted samples of drugs maintained and recommended by the Expert Committee of the Biological Standardization of W.H.O. They represent the fixed units of activity (definite weight of preparation) for drugs. In India, standard drugs are maintained in Government institutions like Central Drug Research Institute, Lucknow, Central Drug Laboratory, Calcutta, etc.
The problem of biological variation must be minimized as far as possible. For that one should keep uniform experimental conditions and assure the reproducibility of the responses.
Methods of Bioassay for Agonists
An agonist may produce graded response or quantal response. Graded response means that the response is proportional to the dose and response may lie between no response and the maximum response. By quantal, it is meant that the response is in the form of "all or none", i.e. either no response or maximum response. The drugs producing quantal effect can be bioassayed by end point method. The drugs producing graded responses can be bioassayed by (1) Matching or bracketing method or
(2) Graphical method.
1. End Point Method: Here the threshold dose producing a positive effect is measured on each animal and the comparison between the average results of two groups of animals (one receiving standard and other the test) is done. e.g. bioassay of digitalis in cats. Here the cat is anaesthetized with chloralose and its blood pressure is recorded. The drug is slowly infused into the animal and the moment the heart stops beating and blood pressure falls to zero, the volume of fluid infused is noted down. Two series of such experiments-one using standard digitalis and the other using test preparation of digitalis is done.
In case, if it is not possible to measure individual effective dose or if animals are not available, fixed doses are injected into groups of animals and the percentage of mortality at each dose level is determined. The percentage of mortality is taken as the response and then the comparison is done in the same way as done for graded response.
2. Matching Method: In this method a constant dose of the test is bracketed by varying doses of standard till the exact match is obtained between test dose and the standard dose.
Initially, two responses of the standard are taken. The doses are adjusted such that one is giving response of approximately 20% and other 70% of the maximum. The response of unknown which lies between two responses of standard dose is taken. The panel is repeated by increasing or decreasing the dose s of standard till all three equal responses are obtained. The dose of test sample is kept constant. At the end, a response of the double dose of the standard and test which match each other are taken. These should give equal responses.
This method has following limitations:
1. It occupies a larger area of the drum as far as tracings are concerned.
2. The match is purely subjective, so chances of error are there and one cannot determine them.
3. It does not give any idea of dose-response relationship.
However, this method is particularly useful if the sensitivity of the preparation is not stable. Bioassay of histamine, on guinea pig ileum is preferably carried out by this method.
3. Graphical method: This method is based on the assumption of the dose-response relationship. Log-dose-response curve is plotted and the dose of standard producing the same response as produced by the test sample is directly read from the graph. In simpler design, 5-6 responses of the graded doses of the standard are taken and then two equiactive responses of the test sample are taken. The height of contraction is measured and plotted against the log-dose. The dose of standard producing the same response as produced by the test is read directly from the graph and the concentration of test sample is determined by the same formula as mentioned before.
The characteristic of log-dose response curve is that it is linear in the middle (20-80%). Thus, the comparison should be done within this range only. In other words, the response of test sample must lie within this range.
Advantage of this method is that, it is a simple method and chances of errors are less if the sensitivity of the preparation is not changed. Other methods which are based on the dose-response relationship include 3 point, 4 point, 5 point and 6 point methods. In these methods, the responses are repeated several times and the mean of each is taken. Thus, chances of error are minimized in these methods. In 3 point assay method 2 doses of the standard and one dose of the test are used. In 4 point method 2 doses of standard and 2 doses of the test are used. In 6 point method 3 doses of standard and 3 doses of the test are used. Similarly one can design 8 point method also. The sequence of responses is followed as per the Latin square method of randomization in order to avoid any bias.
The mean responses are calculated and plotted against log-dose and amount of standard producing the same response as produced by the test is determined graphically as well as mathematically.
Bioassay of Antagonists:
Commonly used method for the bioassay of antagonist is simple graphical method. The responses are determined in the form of the percentage inhibition of the fixed dose of agonist. These are then plotted against the log dose of the antagonist and the concentration of unknown is determined by finding out the amount of standard producing the same effect as produced by the test.
In this method, two responses of the same dose of agonist (sub maximal giving approximately 80% of the maximum response) are taken. The minimum dose of standard antagonist is added in the bath and then the response of the same dose of agonist is taken in presence of antagonist. The responses of agonist are repeated every ten min till recovery is obtained. The higher dose of standard antagonist is added and responses are taken as before. Three to four doses of the standard antagonist are used and then one to two doses of test sample of the antagonist is used similarly. The percentage inhibition is calculated, plotted against log dose of antagonist and the concentration of unknown is determined as usual.





This is such a great resource that you are providing and you it away for free. I love seeing blog that understand the value.
ReplyDeleteParenteral Drugs Supplier In India
Thank you for posting such a great blog. I found your website perfect for my needs. Read About Pharmacy products and services
ReplyDelete